This month, I’ll deal with corrosion – especially the electrolytic effects in LED signs, because humidity is one of the worst wintertime enemies of electric signage. Corrosion, from the Latin corrodere, which means “eating away,” describes how some chemical agents (like acids) can change an object’s constitution and finally dissolve it. I’ll confine myself here to metal corrosion, because it’s the most prevalent type of corrosion in signs.
这个月,我想与大家探讨一下灯罩的腐蚀问题,尤其是电子LED标识,冬季的潮湿的天气是电子标识的最大问题。“Corrosion(一词)”来源于拉丁语“corrodere”一词,含义是“腐蚀、损坏”,用来描述一些化学试剂对其他物质结构和形态的改变。这里我们只探讨金属腐蚀,因为金属是标识行业里最常见的物质。
Rusting iron is the most common form of metal corrosion. However, iron won’t rust automatically; kept dry or in absolutely pure water, it’ll stay shiny forever. But in a humid environment, iron transforms into a brittle, brown-grey substance: rust, chemically called iron oxide (more precisely: a mixture of different iron oxides, oxide-hydrates and/or iron carbonates). To understand corrosion – and prevent it – we need insights into its surface chemistry.
铁锈是金属腐蚀里最常见的形式。但是,铁是不会自己生锈的,在干燥或者完全水下的环境中,它的光泽是不会减弱的。在潮湿的环境中,铁会变得灰褐色脆弱的物质,即我们常说的铁锈,化学中称为氧化铁(更确切的说是不同程度的氧化铁和碳酸铁的混合物)。为了更好地理解和防止腐蚀,我们需要仔细研究铁表面的化学特性。
Reasons for rust
腐蚀的原因

Oxidization (the formation of an oxide compound from metal and oxygen) of a metal is the removal of electrons; the reverse effect, reduction, captures electrons. Every atom has a specified-strength affinity to electrons (i.e., a strength to hold or repel them), sometimes called “electron pressure.”
金属的氧化(主要是氧和金属物质之间发生的化学反应)会减弱金属的导电性。电子有正负之分,他们根据同性相吸,异性相斥的原理运动,由此形成电压。
So what happens if iron is put into water? As said, pure water won’t cause rusting, because it’s electrically neutral and insulating (absolutely pure water doesn’t conduct electricity!). It has no free electrons nor free charged atoms, which are called ions.
如果标识不得不在水中工作的话,如上所述,纯净的水中铁是不会腐蚀的,因为它是电中性的而且是绝缘的(绝对纯净的水是不导电的!)。因为绝对纯净的水中没有自由的电子,无法形成自由电荷,也就是我们常说的电离子。
In contrast, common water always contains dissolved gases and minerals. For example, carbon dioxide (CO²) from the air forms H²CO³, carbonic acid, which in water exists in two separate parts, H+ and CO³- , as electrically charged ions. Chemists say the carbonic-acid molecule dissociates into ions. When iron and water interact, the iron attracts the CO3- ions, generates surplus electrons, and becomes negatively charged.
与此相反的是,普通的水里往往溶解了气体和矿物质,比如空气中的二氧化碳(CO²)在水中会形成H²CO³(碳酸也叫甲二酸,是一种二元弱酸。),碳酸在水中会分解为H+ 和CO³-,形成带电离子。当铁和水接触时,便会发生化学反应,铁会与CO3-发生化合作用,水中会剩余大量的负电离子。

If I immerse copper wire into the water, it tends to repel electrons and attract positive, hydrogen ions. Thus, the copper wire becomes positively charged in water. The iron’s charge, for example, is created by the CO³- ion being pulled to the iron’s surface. It’s broken up into CO² + O-, and the O- is discharged to form FeO, plus one electron e-. This electrical field repels further CO³- ions; so reaction stops when the charge is created.
如果在水中放入铜线,它就会排斥离子,吸引带有正电荷的氢离子。因此铜线在水中就是带正电的。铁的变化是由于碳酸离子CO³-吸附到铁的表面,CO³-会分解为CO² +(碳酸根)和O-(负氧离子),负氧离子与铁发生化学反应形成氧化铁。一旦铁放入水中,这个反应就开始进行,直到溶液中的不能进一步分解CO³-。
If we connect a voltmeter between the two immersed materials, we can measure the difference in electron affinity – called electrochemical potential – in the form of electrical voltage (Photo 1). Here, theoretically, 0.06V (iron) – 0.52V (copper) = 0.58V. When no current flows, the charge repels further CO3- ions, and the situation is purely static. Thus, no further oxidization occurs. If the two metals contact each other, the voltage difference becomes zero. Other ions move to the iron surface, and more oxygen gives off electrons. An electric current results between the materials.
如果我们在插进水里的这两种金属之间连接一个电压器就可以测量它们之间的电子亲和力(也叫电化学势)的差距了。没有电流的时候,电荷是排斥CO3-的,水中保持一个相对平衡的状态,没有进一步氧化的发生。此时,如果在它们之间连接一个电压器的话,电压差为零。其他的电子移动到铁表面,水中的氧会不断地释放更多的离子,这些离子也会像铁表面移动,由此形成电流。
Thus, the two different metals in water act as an electric battery. This current remains as long as there are enough ions in the water to attract the surface. Corrosion greatly increases when two different materials are in contact in water. The metal with the more negative, electrochemical potential is dissolved, while the other is preserved. The effect is also called local element. Even if no macroscopic electricity is involved, when in-contact, differing metals get wet, “galvanic corrosion” occurs. The principle of “galvanized” iron (the trade term even if no galvanic reaction occurs between the coating and zinc) is that the zinc is more negative than the iron, and dissolves instead of the iron.
因此,两种不同的导电金属在水里就是一个小型的电源了。只要水中有足够的离子吸附到铁表面,电流就会一直存在。当两种不同的金属在水中互相发生反应时腐蚀会加剧。金属上的负离子越多,电化学势就越大。即使这一过程中没有可见的电子,一旦相互连接的不同种金属受潮,腐蚀就会发生。金属电镀的方式是指用不活跃的锌涂在铁的表面。
Helpful hints
建议

The more the materials differ in their electrochemical potential, the higher the voltage and the faster the corrosion. The more conductive the water, the worse it is (thus seawater or salt spray on boardwalk signs requires special attention). When constructing signs in humid conditions, avoid these material combinations: stainless-steel screws or rivets in galvanized iron; brass screws in aluminum; pig iron and aluminum (Photo 2); and, stainless-steel screws in mild steel.
不同种金属之间的电化学势差距越大,形成的电压就越大,对金属的腐蚀也更快。水的导电性越强,(木板桥上的标识应该注意防护,以免受海水和盐沫的侵蚀)。如果标识是安装在潮湿的环境中,以下这些材料是不得使用的:镀锌铁不锈钢螺丝或铆钉、铝材上的黄铜螺钉、生铁和生铝等活跃金属。
Anodized aluminum generates a very thin layer of electrically isolating, aluminum oxide on the surface, which prevents humidity from attacking the underlying metal. However, in sign construction, this oxide layer may be penetrated (for example, when self-tapping or sheetmetal screws are used), or if the metal structure must electrically be grounded for safety reasons. Here, toothed washers are mandatory, so the oxide layer is definitively broken to make electrical contact. These breaks in the protective layer are very prone to corrosion by humidity. Usually, they must be protected against the elements, but the applied coating (paint) is usually permitted only after the electrical inspection has occurred.
阳极电镀铝会生产一层很薄的电隔离层,表面的氧化铝会阻止空气中的湿气接触下面的金属,防止金属被腐蚀。但在标识安装的过程中,这些氧化层可能被破坏,或者为了安全起见,这些金属必须接地。因此,为了良好的导电性氧化层必须局部打开,带齿垫圈就很有必要了。氧化层下面的金属会因为氧化层的破坏而被腐蚀。通常涂层是用来保护金属免受外界环境的侵蚀,但是涂层一般是在电参数检查之后才被允许。
Our discussion has covered only electricity generated by different materials in humidity, but it hasn’t focused on electric signs. LEDs in signs have posed new problems for the industry, because most LEDs operate on direct current (DC), compared to old-style electric signs, which worked mainly on alternating current (AC).
我们只讨论了两种不同的材料在潮湿的环境中产生电流的情况,LED标识又为标识行业提供了新的问题,LED以前用的是交流电,现在绝大多数用的都是直流电。
LED problems
LED面临的问题
Although most LED circuits operate on a low voltage (12- or 24VDC), it’s still much higher than the electrochemical potentials (EP) of the materials involved (for a quick EP reference chart for numerous metals, go to www.thelen.us/1galv.php). So if electrical circuits, contacts or wires are exposed to humidity, the DC greatly increases the strength of the ion movement.
LED需要的电压只有12或者24V的直流电,但比金属的电化学势高。如果电路、电线暴露在潮湿的环境中,直流电会加强离子的运动。

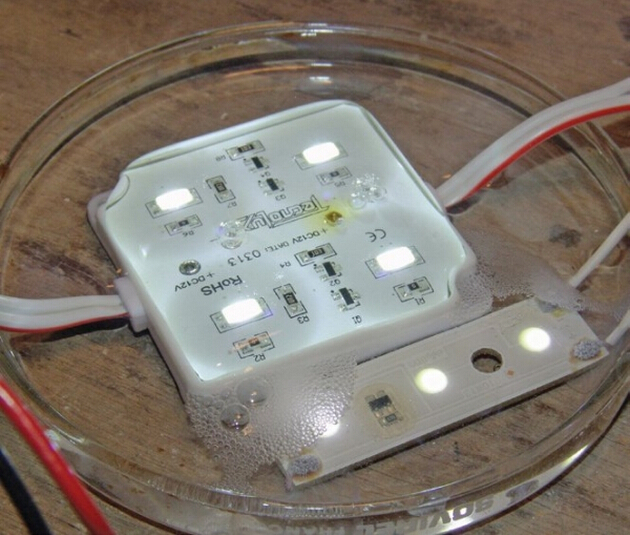
To experiment, I took two common, low-cost LED modules. One had potting shallow enough to expose the screws that connect the circuit board to the wires. In the other, the circuit board was coated with varnish, and the components were additionally covered by a drop of polymer, but the wire solder points were exposed. Both run on 12VDC.
为了证明这一点,我做了一个实验。取两个低压的LED模组,一个模组与电路板相连,并充分暴露在空气中,电路板上涂有清漆。另一个模塑的其他部件用一滴高分子聚合物覆盖,但焊接点暴露在外面。两个模组都在12V的直流电压下运行。
To create corrosive effects, I applied a few drops of tap water on top of the modules. As soon as I switched on the LEDs, you could see gas bubbles developing on the negatively charged wire. Less than eight minutes later, severe corrosion and conductive material surrounded the positive conductors, while the negative ones were heavily pitted and nearly destroyed. DC electricity accelerates corrosion.
为了让它们快速发生腐蚀反应,我在模组上撒了几滴自来水。此时,只要我一打开模组电源的开关,在带负电荷的电线上你可以看到有雾气产生。等七八分钟后,导电性好的那一个模组腐蚀更严重,导电性不好的模组上有很严重的斑点,模组近乎损坏。直流电加速了腐蚀的速度。
With alternating current, the ions would simply be pushed forward, and, when polarity reversed, back in the next fraction of a second. Hence, AC won’t cause the extreme corrosion DC does. Remember, standard acrylic can absorb up to 10% of its own weight in water. Thus, acrylic enclosures don’t make a circuit waterproof!
而在交流电中,离子只是简单地根据“同性相斥,异性相吸”原理移动,一旦两极的性质发生变化,离子移动的方向也瞬间变化。因此,直流电可以引起严重的腐蚀,而交流电不会。你要知道,一般亚克力材料在水中会由于浮力的存在而减轻10%的重量,它的表面也不需要额外的防水材料的保护。

Here’s some advice for outdoor LED signs:
• Prevent rain or condensed water from accumulating in a channel letter or sign box. Make sure drain holes are large enough not to become obstructed by debris.
• If stranded wires carry DC power, they should be watertight lengthwise.
• Printed circuit boards, including all connections, should be potted or coated with a material impermeable to water and water vapor.
• Don’t use exposed conductors (like support rails) to supply DC voltage.
• And, make sure connector blocks inside channel letters or sign boxes are individually waterproofed – don’t use wire nuts.
下面是户外LED标识防护的几点意见:
·发光字或者灯箱标识中要注意防雨以及泠凝水。确保通风孔足够大,不至于被碎屑之类的东西阻塞。
·如果铁丝中是直流电,那就要严格防水了。
·电镀线路板包括所有的连接口都应该密封或者涂上一层防水涂层。
·禁止使用外露的材料运输直流电。
·确保连接板在发光字和灯箱的内部,并且涂有防水材料,禁止使用金属丝。